
- Show results for
- Share
What Is Chromatography? GC vs HPLC. Supelco GC & HPLC Columns. How to Choose a Capillary Column?

Table Of Contents
- Introduction
- What Is Chromatography? GC, LC, and HPLC
- Gas Chromatography vs High-Performance Liquid Chromatography
- Supelco GC Columns. A Capillary Column Selection Guide
- Ascentis® HPLC Columns
- Final Thoughts
Introduction
Chromatography is a widely used technique in chemistry. In this article, you will discover what chromatography is and learn about the difference between liquid chromatography and gas chromatography. You will also find out about Supelco GC columns (and how to choose a capillary column) and Ascentis® HPLC columns.
What Is Chromatography? GC, LC, and HPLC
Chromatography is the process of separating the components of a mixture. The mixture is dissolved in a substance that is called the mobile phase, which carries it through a second substance - the stationary phase. The components of the mixture move through the stationary phase at different speeds. As a result, they separate from one another. The nature of the specific mobile & stationary phases determines which substances travel more quickly/slowly. Such travel time is called the retention time.
The Column Chromatography Procedure

Gas chromatography (GC) is an analytical technique for separating and analyzing volatile & semi-volatile compounds in mixtures. This technique combines excellent resolving power with speed and sensitivity. Applications include environmental, chemical, petroleum, pharmaceutical, as well as food & beverage. How does GC work? Look at the diagram below.
The GC Process
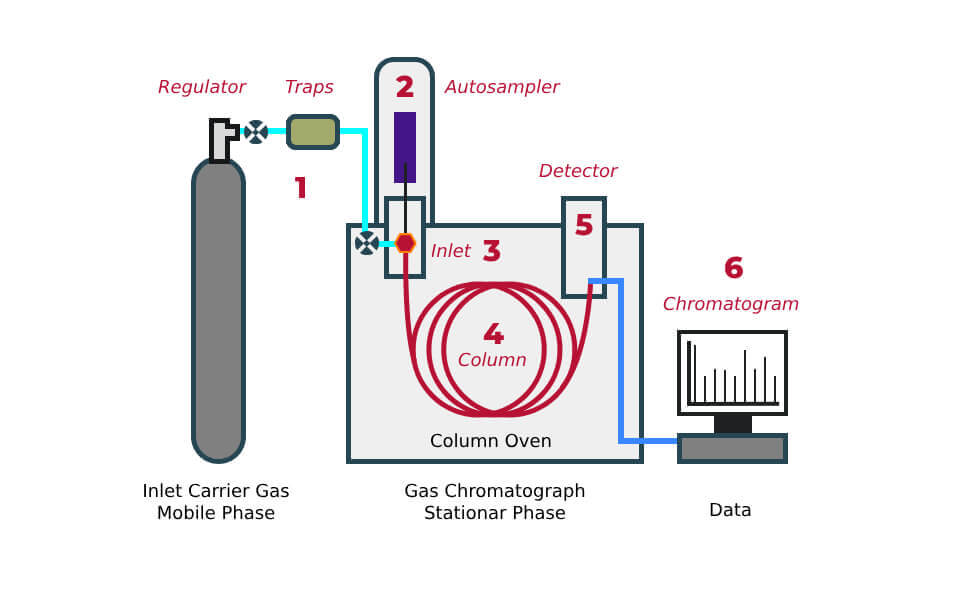
- A. The sample is first introduced into the gas chromatograph, with a syringe most commonly from a liquid autosampler (2).
- B. The sample is injected into the GC inlet (3) through a septum that enables the injection of the sample mixture without losing the mobile phase. The analytical column (4) is connected to the inlet. This column is a long, narrow fused silica or metal tube containing the stationary phase coated on the inside walls.
- C. The column is held in the column oven, which is heated during the analysis to elute the compounds of different boiling points.
- D. The column’s outlet is inserted into the detector (5). It responds to the chemical components eluting from the column to produce a signal.
- E. The signal is recorded by the acquisition software on a computer to produce a chromatogram (6). If necessary, the software can quantify or produce an amount of the compound referencing a known amount of the compound.
Liquid chromatography (LC) is a technique for separating the components of a mixture in which the mobile phase is a liquid.
High-performance liquid chromatography (HPLC) is a technique used for separating, identifying, and quantifying specific components in mixtures. HPLC is faster than traditional LC. High-quality analytical HPLC columns will help you achieve excellent results. How does HPLC work?
The HPLC Process

The solvent that separates the components in a liquid sample for the HPLC analysis is known as the mobile phase. It is delivered to a separation column called the stationary phase, and to the detector at a stable flow rate controlled by the solvent delivery pump. The sample (a certain amount of it) is injected into the column and the compounds in the sample are separated. These compounds are detected by a detector downstream of the column. Each compound is identified and quantified.
Gas Chromatography vs High-Performance Liquid Chromatography
GC or HPLC? Let’s consider the differences.
GC vs HPLC: Key Distinctions
| GC | HPLC | |
|---|---|---|
| Some Things You Will Need |
A long & thin column, carrier gas & gas containers are needed. | A short & wide column, a pressure pump & solvents are required. |
| Mobile Phase | Inert/Unreactive Gas | Liquid (Solvent) |
| Separation Method | The separation depends on the volatility of each compound. Molecules that are more volatile move faster through the column toward the mobile phase. Less volatile molecules move more slowly, they interact more with the stationary phase. | The separation is determined by each compound’s interaction with the mobile & stationary phases and its polarity in relation to them. Components with higher polarity will be more attracted to the mobile phase, and move through the column faster. Less polar components will be attracted to the stationary phase and move more slowly. |
Supelco GC Columns. A Capillary Column Selection Guide
Supelco offers a wide range of analytical products that are designed to meet laboratory needs.
Supelco GC columns are reliable and available in different sizes. They are perfect for successful gas chromatography. The mobile phase drives the gaseous compounds through the heated column coated with a solid/liquid stationary phase. The differences in the chemical & physical properties of the vaporized compounds as well as their interactions with the stationary phase are the basis of the separation process.
A GC capillary column is a popular type that comes with its stationary phase being coated on its inner wall. Such columns are often preferred over the packed options as they need smaller sample amounts for the chromatography process.
How to Choose a Capillary Column?
An effective chromatographic separation begins with the right column choice. Its selection should be based on the following factors: stationary phase, column internal diameter, film thickness, and column length. Let’s consider them in more detail.
Step 1
Stationary Phase
A stationary phase is the film coated on the capillary column’s inner wall and should be chosen depending on the application. The basis of the separation process includes the differences in the chemical & physical properties of injected organic compounds as well as their interactions with the stationary phase. If the strength difference of the analyte-phase interactions is considerable for two compounds, one is retained longer than the other. The retention time is a measure of such analyte-phase interactions.
Modifying the chemical characteristics of the stationary phase affects its physical properties. 2 compounds that fail to separate on one stationary phase might separate on another one of a different chemistry if the difference in the analyte-phase interactions is considerable. Note that each phase provides a unique combination of interactions for each chemical class of analytes.
Phase Polarity
Phase selection is crucial in choosing a capillary column, as it determines the column’s ability to separate the components of a sample. A non-polar column is recommended for the analyses of non-polar compounds. Polar columns are preferred for the separation of polar compounds.
Bonded & Non-Bonded Phases
Bonded phases are immobilized/chemically bonded within the tubing. Non-bonded phases are coated on the wall. Typically, a bonded phase is preferred as there is less bleed during use, it is possible to use higher temperatures, and, if necessary, it can be rinsed with solvents to remove accumulated non-volatile materials. If a bonded phase is unavailable, a stabilized phase may be an option. These phases, while not as permanent as bonded phases, offer better thermal stability than non-bonded phases.
Step 2
Column Internal Diameter
The range of available capillary column internal diameters (I.D.) allows a balance between efficiency (number of theoretical plates) and sample capacity (the amount of a sample component that can be applied without overloading the peak). The best internal diameter depends on the analytical needs.
Let’s consider the effects of column internal diameter on efficiency & sample capacity. Look at the table below:
| Internal Diameter (mm) | Efficiency: Plates/Meter (N/m) | Efficiency: Total Plates (N) | Capacity Each Analyte (ng) |
|---|---|---|---|
| 0.53 | 1,300 | 39,000 | 1000–2000 |
| 0.32 | 2,300 | 69,000 | 400–500 |
| 0.25 | 2,925 | 87,750 | 50–100 |
| 0.20 | 3,650 | 109,500 | <50 |
| 0.18 | 4,050 | 121,500 | <50 |
| 0.10 | 7,300 | 219,000 | <10 |
Theoretical values for 98 ft (30 m) long columns, calculated with k = 6.00 & 85% coating efficiency.
Columns with a 0.25 mm internal diameter offer a good balance between efficiency and sample capacity, making them suitable for most applications. A 0.25 mm internal diameter is most commonly used for capillary GC columns. Columns with smaller or larger internal diameters, on the other hand, allow for optimization of efficiency or sample capacity, depending on the specific needs of the application.
The efficiency of capillary columns increases as their internal diameter decreases. Sample capacity increases as the internal diameter of the column increases.
Step 3
Film Thickness
Most columns with a 0.25 mm internal diameter have a film thickness of 0.25 or 0.50 μm. The most suitable film thickness can vary based on the specific requirements of the application.
The film thickness decrease results in sharper peaks (may increase resolution) and reduced column bleed as well as increased maximum operating temperature of the column. On the other hand, decreasing film thickness will lead to increased analyte interaction with the tubing wall, and decreased analyte capacity. Another thing to know is that analytes will elute with shorter retention times and at lower temperatures. Thinner film columns are best suited for analytes with high boiling points.
The film thickness increase pros include reduced analyte-tubing interaction & increased sample capacity. The cons are increased peak widths (may reduce resolution), increased column bleed, and a reduced max operating temperature for the column. In addition, increasing film thickness results in increased analyte retention (may increase resolution, for compounds with low k) as well as increased elution temperature. Thicker film columns should be used for analytes with low boiling points.
Step 4
Column Length
A 98 ft (30 m) column typically offers an optimal balance of resolution, analysis time, and also required column head pressure, as shown in the table below. However, certain applications may require a different column length.
| Column Length (m) | Inlet Pressure (psi) | Peak 1 Retention (min) | Peak 1/2 Resolution (R) | Efficiency: Total Plates (N) |
|---|---|---|---|---|
| 15 | 5.9 | 8.33 | 0.8 | 43,875 |
| 30 | 12.0 | 16.68 | 1.2 | 87,750 |
| 60 | 24.9 | 33.37 | 1.7 | 175,500 |
Theoretical values for columns with a 0.25 mm internal diameter with 85% coating efficiency, 293°F (145 °C) isothermal analyses, helium at 21 cm/sec, k (peak 1) = 6.00
A longer column offers a greater resolution, however, it increases back pressure. Pay attention that resolution only increases according to the square root of the column length. A shorter column is suitable for applications where a great resolution is not necessary. If the internal diameter of the column is decreased along with the length, resolution can be maintained or even increased.
Ascentis® HPLC Columns
Ascentis® HPLC Columns are designed with the following concepts in mind: efficiency, retention, and selectivity. Ascentis® bonded phases have a broad range of selectivities. The chance that one (or more) Ascentis® phase will accomplish any small molecule HPLC separation is high. Ascentis® solutions are suitable for various HPLC applications, even highly sensitive trace-level analyses.
Key Features Of The Ascentis® Family:
- High purity, type B silica for inertness, reproducibility, as well as stability.
- Cutting-edge bonding processes, optimizing bonded phase coverage & maximizing stability, while minimizing bleed & unwanted secondary interactions.
- Extensive range of bonded phase chemistries & bare silica.
- Phases with improved polar compound retention.
- Compatibility with LC-MS and all modern sensitive instruments & methods.
- Scalable selectivity from analytical to preparative applications.
- High surface area silica for increased preparative loading capacity.
Phosphate buffers are commonly used in HPLC applications, however, at high pH, they are aggressive, potentially causing silica dissolution, stripping of phase, and voiding in columns. Typically, they are not recommended to be used at high pH conditions.
Ascentis® columns are stable within the pH 2-8 range. However, under some conditions, such as lower temperatures, Ascentis® C18, C8, and RP-Amide can be used with mobile phase conditions at pH 1.5 & pH 10, even with aggressive mobile phase conditions with phosphate & methanol.
Final Thoughts
After reading this article, you know what the principle of chromatography is, and the difference between GC, LC, and HPLC. Liquid chromatography vs gas chromatography? Now you know which one is best for your application. In addition, you understand the benefits of Supelco Ascentis® HPLC columns and how to choose a capillary column. On Prime Lab Med, there is a wide range of Supelco HPLC columns and GC columns. Shop with us!
